Whitefly
General Information
Whiteflies are a major pest of greenhouse crops including tomatoes, cucumbers, and many ornamental species, particularly poinsettia, gerbera, and a number of spring crops. Many weed species are also hosts of whiteflies and often serve as sources of infestations.
Description
Adult whiteflies are small, winged, white insects about 1.5-2 mm long.
Their eggs are small and elongated and stand on their end on the undersides of leaves. They are light green when first laid, but change to a darker colour after a few days (Figure 1). They are best seen with a handlens or microscope.
After the eggs hatch, the first immature life stage moves a short distance before settling down to feed. It then remains in the same position until the adult emerges.
Whitefly Species
There are 2 whitefly species of primary concern to greenhouse growers in Canada: the greenhouse whitefly (GWF) (Trialeurodes vaporariorum) and the silverleaf (or sweetpotato) whitefly (Bemisia argentifolii; also known as B. tabaci). This species will be referred to simply as Bemisia on this site for reasons outlined further below. In some greenhouse production regions in other countries, there may be other local whitefly species that can also become pests of greenhouse crops.
GWF and Bemisia adults look very similar, although there are some differences:
Bemisia is slightly smaller than GWF, and its body more yellow in colour. However, body colour can be difficult to see because of the waxy white wings which cover the body.
At rest, Bemisia holds its wings tent-like above its body, while the GWF holds them flatter and more parallel to the surface on which it is resting. This characteristic makes Bemisia look narrower in its body shape, and GWF more triangular when at rest. (Figure 2A, Figure 2B).
The major diagnostic differences between GWF and Bemisia occur in the pupal stage (4th nymphal stage). In fact, for most whitefly species, this life stage is the most important for identification. The GWF pupa is raised off the leaf surface and is surrounded by a fringe of hairs whereas the Bemisia pupa sits flat on the leaf and does not have a fringe1. These features are best seen with a microscope but can also been seen with practice with a 10X hand lens. Bemisia pupae are much more yellow in colour than GWF which are best described as white or cream coloured. (Figure 3A, Figure 3B).
Two species of Bemisia are commonly found in North American greenhouses and there has been considerable discussion since the early 1990’s as to whether they are biotypes, strains or separate species. As of mid-2011, the most recent thinking is that they are separate species and that the Bemisia genus consists of 24 species around the world2, all of which had previously been considered part of a complex of biotypes by many researchers. The species, however, have not yet been named and can only be distinguished by molecular technology; they are in general appearance (even to experts), identical. Because these species are not yet named (June 2011), the biotype designations will be used to explain the history of this insect in greenhouses. The B biotype has been a pest in greenhouses since the mid-to-late 1980s and is found most often on poinsettia (it has also been known as the “poinsettia strain”). The Q biotype is Mediterranean in origin and was found for the first time in North America on poinsettia in 2004. It is reported to rapidly develop resistance to pesticides. Both “biotypes” are now commonly found on poinsettia.
In Ontario, Bemisia is most commonly found on poinsettia. It can establish on other crops, however most growers of poinsettia do not carryover infestations on other crops. Once the poinsettia crop leaves the greenhouse at Christmas, Bemisia are usually no longer an issue. It is uncertain why there is limited transfer to other crops, since Bemisia has a very wide host range, but it may be because it has acclimated to poinsettia which are produced year-round in screened greenhouses, essentially isolating the Bemisia population.
- Figure 1
- Figure 2A
- Figure 2B
- Figure 3A
- Figure 3B
Biology
The life span of adults also depends on temperature and crop. They may live as long as 50 days at cooler temperatures (16-18°C) or less than 10 days at warmer temperatures (28-30°C)1.
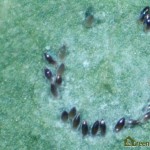
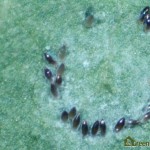
Eggs are laid on the underside of the youngest leaves, and are too small to be seen clearly without the aid of a microscope or hand lens (Figure 4). A female whitefly may lay hundreds of eggs during her lifetime, depending on the whitefly species, the host crop and the temperature1. GWF often lay their eggs in a circular or semi-circular pattern, especially on plants with smooth leaves3(Figure 5).
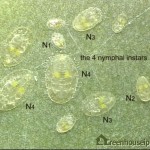
After hatching, the whitefly develops through 4 nymphal stages (or instars) before becoming adults (Figure 6).
The first stage of the immature whitefly (sometimes called the crawler) is flat and scale-like. It moves (crawls) a short distance from the egg before becoming immobile. The whitefly becomes progressively larger through the second, third and fourth stages. The fourth stage (sometimes called the pupal stage, especially when the red eyes of the adult are visible), is the final one from which the adult emerges (Figure 7).
There are differences in the biology of whiteflies depending on the species (GWF or Bemisia), the host plant on which the whitefly is living and environmental factors (especially temperature).
In general terms Bemisia develops more quickly (16-18 days) than GWF (22-26 days) at warmer temperatures (30°C) as might be expected from an insect of tropical origin. GWF on the other hand develops more quickly (31-32 days) than Bemisia (37-39 days) at cooler temperatures (20°C)4.
However, it is also dependent on the host plant on which the whitefly is living5. For Bemisia reared at a constant temperature on different vegetable host plants, the time for it to develop from egg to adult varied between approximately 17 days (on eggplant) to 21 days on bean6.
- Figure 4
- Figure 5
- Figure 6
- Figure 7
Damage
- Damage is caused in a number of ways:
- The piercing-sucking mouthparts of whiteflies allow them to remove sap from the plant which causes a reduction of plant vigour.
- They excrete large amounts of a sugary substance called honeydew. This honeydew promotes the growth of a black sooty mould fungus on plant surfaces, thereby reducing crop quality (Figure 8A, Figure 8B). The sooty mould itself does not damage the plants although in large quantities it can reduce the photosynthetic capacity of the plant.
- Whitefly are responsible for carrying more than 70 plant viruses, the bulk of which are transmitted by Bemisia7. Vegetable crops are much more affected by whitefly transmitted viruses than ornamentals. GWF has been associated with spread of beet pseudo-yellows virus in cucumbers8 (Figure 9).
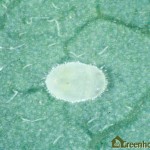
- In ornamental crops, there is a very low threshold for whitefly; their visible presence on its own detracts from the appearance and quality of the crop (Figure 10). Old pupal skins and adults may be found on the underside of lower leaves, which may have symptoms of wilt (Figure 11).
- Figure 8A
- Figure 8B
- Figure 9
- Figure 10
- Figure 11
Management
Monitoring
Implement a routine monitoring program using yellow sticky cards and plant inspections (Figure 12A, 12B).
Whiteflies are found on the undersides of leaves. Adults are attracted to younger leaves for egg-laying (Figure 13) and the nymphal stages mature as the leaves do, so they are found on older leaves.
Some growers use plants that are more attractive to whiteflies than the main crop, as an early detection tool or as a trap plant. Plants such as eggplant (Figure 14) and tobacco can be used for these purposes. Mark infested plants so they can be used as indicators of the success of control strategies.
If considering the use of plants (other than the crop) for monitoring purposes or as trap plants, watch them very closely. If heavy infestations develop, these plants could become a source of further problems rather than a solution. Additionally, monitor them carefully for pests other than whitefly.
React promptly when whitefly adults are observed either on cards or on terminal leaves.
Identify any whitefly species present on the crop.
Cultural Controls
If possible, quarantine new seedlings, transplants, or cuttings until satisfied that they are whitefly and virus-free.
Maintain good weed control inside and outside the greenhouse.
Biological Control
In North America, there are currently a number of commercially available biological control agents for whiteflies, including:
Three parasitic wasps:
Several predators:
- a predatory mite, Amblyseius swirskii
- a small black ladybeetle, Delphastus catalinae
- a predatory bug, Dicyphus hesperus
Insect pathogenic fungus:
- Beauveria bassiana (BotaniGard)
Nematodes:
Physical Control
Sticky Traps: Yellow sticky traps in various forms can be used to trap large numbers of adult whiteflies. Large, yellow, sticky boards or 30 cm wide yellow sticky tapes can be used in ‘hot spots’ (Figure 15) Alternatively, lengths of yellow, sticky tape can be draped between posts along plant rows (Figure 16) Such tapes will also trap thrips, fungus gnats, shore flies. However, they will also attract and catch parasitic wasps (especially under low whitefly populations), so be careful if using sticky tape where flying BCA’s are being used.
Vacuuming: Hand-vacuuming of adults in small areas of ‘hot-spots’ can be very effective in rapidly removing adult whiteflies (Figure 17A, Figure 17B).
Insect barriers: Installation of fine screens over vents and doorways will significantly reduce the movement of outdoor populations into greenhouses. Where whiteflies move in from adjacent field crops (e.g. field tomatoes) in large numbers at certain times of the year (e.g. during harvest), screening should be considered as the first line of defence9,10,11 (Figure 18). (See IPM Basics)
Chemical Control
Whiteflies have shown an ability to develop resistance to many pesticides. Judicious use will extend the useful life of pesticides and at the same time, delay build-up of resistance. Rational and judicious use of pesticides must be used in conjunction with a regular monitoring program, using action thresholds, rotating chemical classes, and at the same time, making use of all available control strategies.
If using pesticides to control whitefly, ensure that they are registered for that use in your country and follow label directions.
For information on the compatibility of pesticides with biocontrol agents, refer to your biocontrol supplier or visit www.Biobest.be or www.Koppert.com.
See two Ontario growers speak about their experience with biological control.
- Figure 12A
- Figure 12B
- Figure 13
- Figure 14
- Figure 15
- Figure 16
- Figure 17A
- Figure 17B
- Figure 18
References
1Malais M.H and Ravensberg W.J. 2003. In Knowing and Recognizing: The biology of glasshouse pests and their natural enemies. Koppert BV, Berkel en Rodenrijs, The Netherlands and Reed Business Information, Doetinchem, The Netherlands.
2De Barro, P.J., Liu, S., Boykin, L.M. and Dinsdale A.B. 2011. Bemisia tabaci: A statement of species status. Annual Review of Entomology. 56: 1-19
3Byrne D.N. and Bellows T.S. 1991. Whitefly biology. Annual Review of Entomology, 36: 431-457.
4Tsueda, H. and Tsuchida, K. 1998. Differences in spatial distribution and life history parameters of two sympatric whiteflies, the greenhouse whitefly (Trialeurodes vaporariorum Westwood) and the silverleaf whitefly (Bemisia argentifolii Bellows and Perring), under greenhouse and laboratory conditions. Appl. Entomol. Zool. 33(3): 379-383.
5Enkegaard, E. 1993. The poinsettia strain of the cotton whitefly, Bemisia tabaci (Homoptera: Aleyrodidae), biological and demographic parameters on poinsettia in relation to temperature. Bulletin of Entomological Research, 83: 535-546.
6Tsai, J.H. and Wang, K. 1996. Development and reproduction of Bemisia argentifolii (Homoptera: Aleyrodidae) on five host plants. Environmental Entomology, 25(4): 810-816.
7Cohen, S. Epidemiology of whitefly-transmitted viruses. In: Whiteflies: Their Bionomics, Pest Status and Management (ed: D. Gerling), Athenaeum Press, Newcastle upon Tyne, pp 211-225
8Duffus, J.E. 1965. Beet pseudo-yellows virus, transmitted by the greenhouse whitefly (Trialeurodes vaporariorum Westwood). Phytopathology 55: 450-453.
9Murphy, G and Ferguson, G. 2000. Screening of greenhouses for insect exclusion. Ontario Ministry of Agriculture, Food and Rural Affairs FactSheet, Agdex 290/626, Order No. 00-021. http://www.omafra.gov.on.ca/english/crops/facts/00-021.htm
10Bell, M.L. and Baker, J.R. 1997. Choose a greenhouse screen based on its pest exclusion efficiency. North Carolina Flower Growers Bulletin, 42(2): 7-13.
11Bethke, J. 1994. Considering installing screening? This is what you need to know. Greenhouse Manager, April, 1994, pp34, 36, 37.
External Links
- http://www.appliedbio-nomics.com
- http://www.biobestgroup.com/
- http://www.koppert.com
- http://www.naturalinsectcontrol.com
- http://www.syngenta-bioline.co.uk/
- http://mrec.ifas.ufl.edu/LSO/bemisia/bemisia.htm
- http://entnemdept.ufl.edu/creatures/veg/leaf/silverleaf_whitefly.htm
- http://www.biocontrol.ucr.edu/bemisia.html
Further Reading
Brown, J. K., Frohlich, D.R. and Rosell, R.C. 1995. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annual Review of Entomology, 40: 511-534
Heinz, K.M., Van Driesche, R.G. and Parrella, M.P. 2004. Biocontrol in Protected Culture (eds: K. M. Heinz, R.G. Van Driesche and M.P. Parrella), Ball Publishing, Batavia, Illinois, 552 pp.
Van Roermund, H.J.W. and van Lenteren, J.C. 1992. The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae) XXXIV. Life-history parameters of the greenhouse whitefly, Trialeurodes vaporariorum as a function of host plant and temperature. Wageningen Agricultural University Papers, 92-3, 102 pp.